In our defence (spelt correctly) all of the above are acceptable, except the microwave. Reasons being that a) the microwave doesn't boil it evenly, and you get pockets of mega heated water that bubble up and splash up in the microwave, then drip off the manky ceiling of the microwave and into your cup. B) microwaves stink. I don't know anyone that uses one for anything other than popcorn or melting butter. But if you're using it to cook as well.... 🤢
Clean out your fuckin microwaves.
Convection currents stir the water automatically, heating it unevenly doesn't matter. A stovetop also heats water unevenly.
Stop microwaving fucking fish you dirty bastards. I will punt any mf who microwaves fish into the fuckin Gehenna.
Convection currents don’t stir water in a microwave because the heat source isn’t on the bottom. That’s the difference. You get temperature stratified water where the surface is hotter than the bottom of the cup and they don’t naturally mix.
Of course, here in America, we have this incredible technology called a spoon. Pull that bad boy out, give a little stir, problem solved.
Convection currents don't need the heat source to be directly at the bottom to stir the liquid, it just needs cold water to be on-top of hot, because cold is more dense.
Microwaves don't really heat top to bottom either, it's shooting waves through the body of the water and even the cup, directly exciting a bunch of individual H2O atoms in hot spots where the microwaves peak at, (e.g. the actual microwaves not the name of the machine) heating the liquid very unevenly. The wave could very much be heating a fraction of the top, middle, and bottom at different points in 3d space. it just depends on the peak of the micro-waves.
I'm well aware of temperature stratification. It doesn't happen in a microwave in the same way.
Micro waves don't heat purely the top surface, they penetrate the entire waters body creating super-heated localized hotpots that shift the water around from Convection currents because the hotter more excited water atoms are less dense than the colder less excited water atoms above them spreading temperature out from those hotspots.
Temperature stratification only comes into play if there's no nucleation point, in which you get this.
Also, your link is dead.
I'm well aware of temperature stratification. It doesn't happen in a microwave.
It empirically does. We can argue about the theory all day but the research says microwaves produce stratified temperature gradients when heating liquids. However, I’d point out that, in atmosphere, when we have localized hot spots the warm air can effectively travel in bubbles without significant mixing for quite some distance. There seems to be a similar phenomena at work when microwaving liquids.
See the screenshot below.
I pulled this from “Multiphysics analysis for unusual heat convection in microwave heating liquid” published in 2020 in AIP Advances.
Relevant excerpts:
“ Usually, the fluidity of liquids is considered to make the temperature field uniform, when it is heated, because of the heat convection, but there is something different when microwave heating. The temperature of the top is always the highest in the liquid when heated by microwaves.”
“ The experimental results show that when the modified glass cup with 7 cm metal coating is used to heat water in a microwave oven, the temperature difference between the upper and lower parts of the water is reduced from 7.8 °C to 0.5 °C.”
“According to the feedback from Midea (microwave appliance makers), when users use the microwave oven to heat liquids such as milk or water, the temperature at the top of the liquid will be significantly higher than the temperature at the bottom.”
That's not really showing temperature stratification which is a more extreme separation of temperature from surface heating :
That's just showing that the hottest atoms gather to the top, which btw, proves Convection currents.
Again, microwaves don't heat purely the surface :
(a) Schematic diagram of convection in the bottom heated liquid and unusual convection in the microwave heated liquid and (b) schematic diagram of convection in liquids heated by microwaves with the modified glass.
The modified glass is just diverting the hotpots to the bottom to make the convection less "unusual".
They aren't claiming that convection doesn't accrue, only that it's "unusual convection" resulting in less even heating like that of thermal stratification, not literal thermal stratification where the layers have separate convection currents that prevent mixing all together.
That's not really showing temperature stratification which is a more extreme separation of temperature from surface
I think the definition you are using is far too restrictive, in many contexts temperature stratification simply refers to a situation where you get temperature gradients across a fluid with the warmer fluid gathered near the top of the body. For example, in a factory you will often have “destratification” fans operating because warm air from equipment rising to the ceiling results in a temperature gradient from floor the ceiling.
It is not a phenomena exclusive to surface heating.
That's just showing that the hottest atoms gather to the top, which btw, proves Convection currents.
Yes. My point was not to establish that convection is magically absent from fluids in microwaves, but to establish that it differs significantly from stovetop heating. Convection currents in stovetop heating create a strong stirring action that produces a substantially uniform temperature. Microwaves do not create the same stirring action and this produce a significant nonuniform temperature gradient.
The modified glass is just diverting the hotpots to the bottom to make the convection less "unusual".
Clearly. They make the heating more akin to a stovetop, which is really the point here.
They aren't claiming that convection doesn't accrue, only that it's "unusual convection" resulting in less even heating like that of thermal stratification, not literal thermal stratification where the layers have separate convection currents that prevent mixing all together.
Once again, you are using a definition of thermal stratification that is far too specific. However, arguing over it is really just being pedantic because the core point at issue here is whether or not heating a cup in a microwave or a stovetop produce the same final product. They do not unless you apply some mechanical agitation to mix it up.
using a definition of thermal stratification that is far too specific
I'm using the textbook definition of which there are at-least three distinct layers that prevent mixing due to distinctly separate convection currents separated by the thermocline layer.
While the top has a considerable difference of ~18F at 95-113F, the rest is pretty evenly 77-86F.
By you're less strict definition, after applying conventional bottom-up heating, Thermal stratification would also occur in this 2 layer form just by letting it sit and settle for a bit as the hotter atoms rise to the top due to their lower density creating a distinct hotter top with the rest holding a pretty even temp.
Matter of fact, the steam is just moister in the air combining with the hottest water atoms that are yeeting themselves out from the surface as vapor.
Search the literature for thermal stratification. There are many contexts where it is used outside of lakes and other large bodies of water, many of which do not consist of three distinct layers. Hell, the paper I cited SPECIFICALLY refers to the temperature gradient in the microwaved glass as “stratification”.
If you can’t understand the use of a term outside your specific area of expertise then thats honestly a you problem and that’s all I can say on that.
If the heating methods were as similar as you say, there wouldn’t be hundreds of publications accepted to various journals across the past two decades investigating the problem where microwaves produce a strong temperature gradient between the top and bottom of a body of liquid. It’s a well known process control problem.
I guess this does count as a more gradual example of thermal stratification where 35C is the thermocline layer.
However, by this definition, thermal stratification would still occur after applying conventional bottom-up heating by letting it sit and settle for some time allowing gravity to sort by density resulting in a very similar stable thermal stratification pattern.
You'd have to be constantly mixing or never take of the heat source to prevent this stable thermal stratification pattern from occurring.
I don’t think that is the case. A stratified fluid, in the absence of continued energy exchange with the outside environment, will eventually reach a homogenous temperature distribution due to diffusion.
That said, even if you are were correct, in the context of brewing tea we would only have a few minutes of brew time in which the stratification would have an impact on the extraction.
You gotta clean the microwave regularly like anything else. There are reasons why I would probably use my stove top over my microwave to boil water (though I do use a microwave to make tea when I just want a single serving), but your points about water splashing up everywhere and dripping down off of disgusting interior surfaces of the microwave sound a lot like operator error.
If you're microwaving water for more than 2-4 minutes you're doing something very very wrong.
1m 30s to 2mins is already enough for 1 coffee cup worth of water to reach boiling temp in the majority of microwaves.
I'm just imagining @Mr_Blott@lemmy.world microwaving a cup of water for way too long to absolutely volcanic results and then throwing up his hands in disgust before walking away from the swampy microwave without bothering to clean the mess up like a scene out of some infomercial for a device that solves microwave issues that don't exist lol
Like I ever microwaved a cup of water 😂 I'm not a fucking barbarian lmao
Yeeeeah, that's not how microwaved water works. If there IS any temperature differential, the movement of the water quickly evens it out. By the time you're dropping your tea in, it's even.
As far as microwaves being stinky, that's a you thing, bud. My microwave smells fine.
Which is why it's important to put the teabag in the water before microwaving it.
Or just like gently stir the water when it comes out of the microwave. You'd really have to overcook the fuck out of the water to create a risk of superheated water explosions. Tea should be slightly below boiling anyway.
Which is why it's important to put the teabag in the water before microwaving it.
I know you are trying to bait me and I'm not going to fall for it
I thought tap water had enough particulate in it by itself?
Usually it does, but then again there are places where people don't drink the tap water.
Just go the whole hog: put the teabag in the bottle of water and microwave that.
In our defence (spelt correctly) all of the above are acceptable, except the microwave. Reasons being that a) the microwave doesn't boil it evenly, and you get pockets of mega heated water that bubble up and splash up in the microwave, then drip off the manky ceiling of the microwave and into your cup. B) microwaves stink. I don't know anyone that uses one for anything other than popcorn or melting butter. But if you're using it to cook as well.... 🤢
Convection currents don’t stir water in a microwave because the heat source isn’t on the bottom. That’s the difference. You get temperature stratified water where the surface is hotter than the bottom of the cup and they don’t naturally mix.
Of course, here in America, we have this incredible technology called a spoon. Pull that bad boy out, give a little stir, problem solved.
Convection currents don't need the heat source to be directly at the bottom to stir the liquid, it just needs cold water to be on-top of hot, because cold is more dense.

Microwaves don't really heat top to bottom either, it's shooting waves through the body of the water and even the cup, directly exciting a bunch of individual H2O atoms in hot spots where the microwaves peak at, (e.g. the actual microwaves not the name of the machine) heating the liquid very unevenly. The wave could very much be heating a fraction of the top, middle, and bottom at different points in 3d space. it just depends on the peak of the micro-waves.
I mean, it’s not really a matter of debate TBH. There are a number of peer reviewed journal articles documenting the temperature stratification. Here is one source, where the authors attempt to create a special cup to heat the water more evenly.
I'm well aware of temperature stratification. It doesn't happen in a microwave in the same way.
Micro waves don't heat purely the top surface, they penetrate the entire waters body creating super-heated localized hotpots that shift the water around from Convection currents because the hotter more excited water atoms are less dense than the colder less excited water atoms above them spreading temperature out from those hotspots.
Temperature stratification only comes into play if there's no nucleation point, in which you get this.
Also, your link is dead.
It empirically does. We can argue about the theory all day but the research says microwaves produce stratified temperature gradients when heating liquids. However, I’d point out that, in atmosphere, when we have localized hot spots the warm air can effectively travel in bubbles without significant mixing for quite some distance. There seems to be a similar phenomena at work when microwaving liquids.
See the screenshot below.
I pulled this from “Multiphysics analysis for unusual heat convection in microwave heating liquid” published in 2020 in AIP Advances.
Relevant excerpts:
“ Usually, the fluidity of liquids is considered to make the temperature field uniform, when it is heated, because of the heat convection, but there is something different when microwave heating. The temperature of the top is always the highest in the liquid when heated by microwaves.”
“ The experimental results show that when the modified glass cup with 7 cm metal coating is used to heat water in a microwave oven, the temperature difference between the upper and lower parts of the water is reduced from 7.8 °C to 0.5 °C.”
“According to the feedback from Midea (microwave appliance makers), when users use the microwave oven to heat liquids such as milk or water, the temperature at the top of the liquid will be significantly higher than the temperature at the bottom.”
That's not really showing temperature stratification which is a more extreme separation of temperature from surface heating :
That's just showing that the hottest atoms gather to the top, which btw, proves Convection currents.
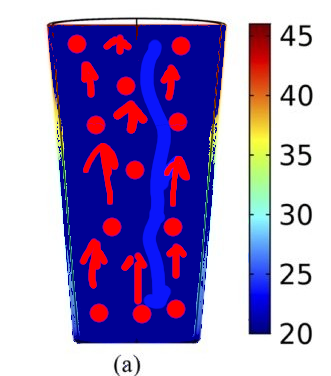
Again, microwaves don't heat purely the surface :

The modified glass is just diverting the hotpots to the bottom to make the convection less "unusual".
They aren't claiming that convection doesn't accrue, only that it's "unusual convection" resulting in less even heating like that of thermal stratification, not literal thermal stratification where the layers have separate convection currents that prevent mixing all together.
I think the definition you are using is far too restrictive, in many contexts temperature stratification simply refers to a situation where you get temperature gradients across a fluid with the warmer fluid gathered near the top of the body. For example, in a factory you will often have “destratification” fans operating because warm air from equipment rising to the ceiling results in a temperature gradient from floor the ceiling.
It is not a phenomena exclusive to surface heating.
Yes. My point was not to establish that convection is magically absent from fluids in microwaves, but to establish that it differs significantly from stovetop heating. Convection currents in stovetop heating create a strong stirring action that produces a substantially uniform temperature. Microwaves do not create the same stirring action and this produce a significant nonuniform temperature gradient.
Clearly. They make the heating more akin to a stovetop, which is really the point here.
Once again, you are using a definition of thermal stratification that is far too specific. However, arguing over it is really just being pedantic because the core point at issue here is whether or not heating a cup in a microwave or a stovetop produce the same final product. They do not unless you apply some mechanical agitation to mix it up.
I'm using the textbook definition of which there are at-least three distinct layers that prevent mixing due to distinctly separate convection currents separated by the thermocline layer.
While the top has a considerable difference of ~18F at 95-113F, the rest is pretty evenly 77-86F.
By you're less strict definition, after applying conventional bottom-up heating, Thermal stratification would also occur in this 2 layer form just by letting it sit and settle for a bit as the hotter atoms rise to the top due to their lower density creating a distinct hotter top with the rest holding a pretty even temp.
Matter of fact, the steam is just moister in the air combining with the hottest water atoms that are yeeting themselves out from the surface as vapor.
Search the literature for thermal stratification. There are many contexts where it is used outside of lakes and other large bodies of water, many of which do not consist of three distinct layers. Hell, the paper I cited SPECIFICALLY refers to the temperature gradient in the microwaved glass as “stratification”.
If you can’t understand the use of a term outside your specific area of expertise then thats honestly a you problem and that’s all I can say on that.
If the heating methods were as similar as you say, there wouldn’t be hundreds of publications accepted to various journals across the past two decades investigating the problem where microwaves produce a strong temperature gradient between the top and bottom of a body of liquid. It’s a well known process control problem.
I guess this does count as a more gradual example of thermal stratification where 35C is the thermocline layer.
However, by this definition, thermal stratification would still occur after applying conventional bottom-up heating by letting it sit and settle for some time allowing gravity to sort by density resulting in a very similar stable thermal stratification pattern.
You'd have to be constantly mixing or never take of the heat source to prevent this stable thermal stratification pattern from occurring.
I don’t think that is the case. A stratified fluid, in the absence of continued energy exchange with the outside environment, will eventually reach a homogenous temperature distribution due to diffusion.
That said, even if you are were correct, in the context of brewing tea we would only have a few minutes of brew time in which the stratification would have an impact on the extraction.
You gotta clean the microwave regularly like anything else. There are reasons why I would probably use my stove top over my microwave to boil water (though I do use a microwave to make tea when I just want a single serving), but your points about water splashing up everywhere and dripping down off of disgusting interior surfaces of the microwave sound a lot like operator error.
If you're microwaving water for more than 2-4 minutes you're doing something very very wrong.
1m 30s to 2mins is already enough for 1 coffee cup worth of water to reach boiling temp in the majority of microwaves.
I'm just imagining @Mr_Blott@lemmy.world microwaving a cup of water for way too long to absolutely volcanic results and then throwing up his hands in disgust before walking away from the swampy microwave without bothering to clean the mess up like a scene out of some infomercial for a device that solves microwave issues that don't exist lol
Like I ever microwaved a cup of water 😂 I'm not a fucking barbarian lmao
Yeeeeah, that's not how microwaved water works. If there IS any temperature differential, the movement of the water quickly evens it out. By the time you're dropping your tea in, it's even.
As far as microwaves being stinky, that's a you thing, bud. My microwave smells fine.
Really the only danger in using a microwave to boil water is superheating if there are no nucleation sites in the mug.
Which is why it's important to put the teabag in the water before microwaving it.
Or just like gently stir the water when it comes out of the microwave. You'd really have to overcook the fuck out of the water to create a risk of superheated water explosions. Tea should be slightly below boiling anyway.
I know you are trying to bait me and I'm not going to fall for it
I thought tap water had enough particulate in it by itself?
Usually it does, but then again there are places where people don't drink the tap water.
Just go the whole hog: put the teabag in the bottle of water and microwave that.